COVID-19 Trial Now Enrolling Participants
Open to adults who
- Tested positive for COVID with symptoms starting within the last 5 days and
- Aged 18–49 years with chronic condition(s) or
- 50+ years regardless of health status
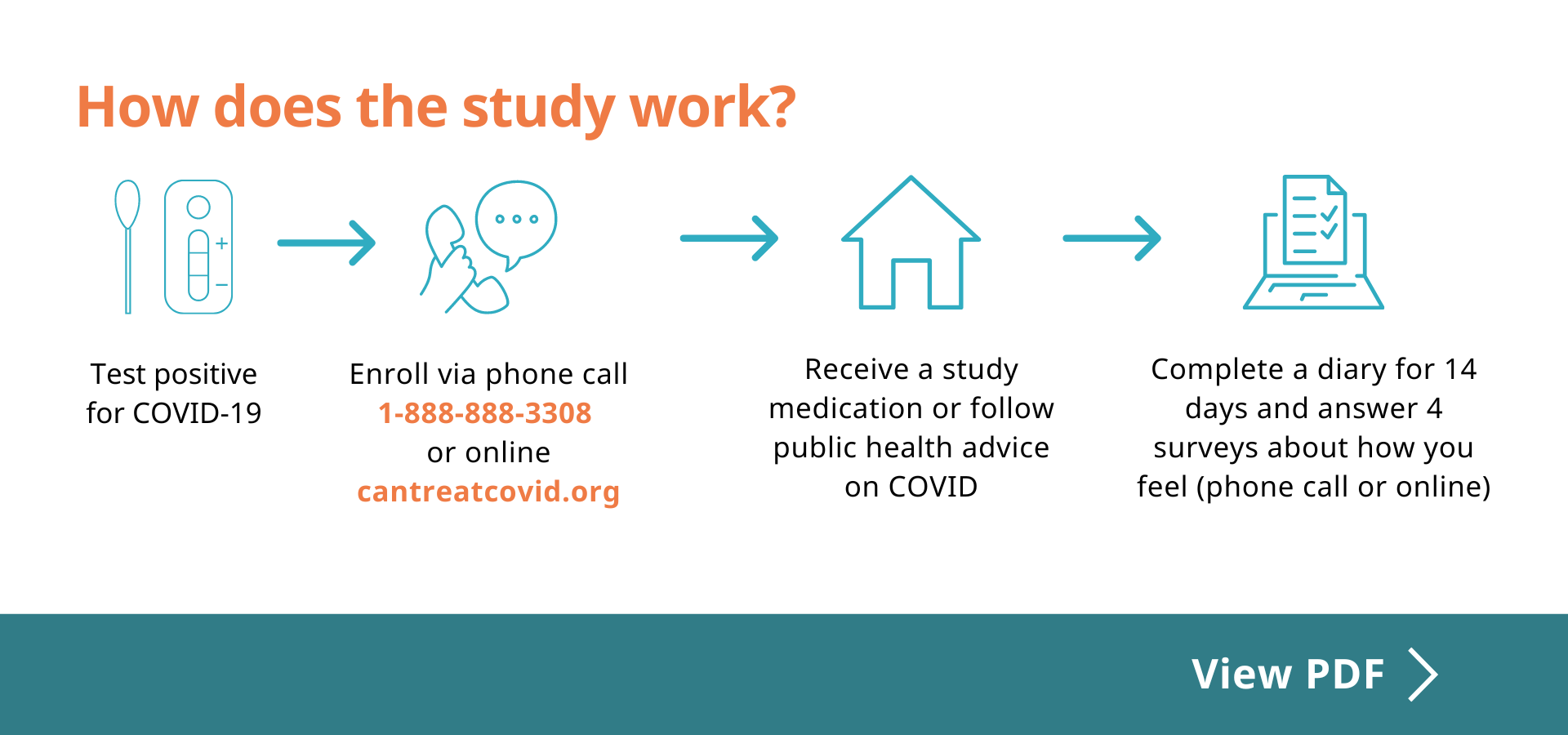
Eligible participants will
- Take a COVID medication approved by Health Canada or follow public health advice on COVID
- Complete an online diary from Day 1 to 14
- Complete a survey on Day 21, 28 and 90 and Week 36
- Receive $30 for each follow-up
Participation is voluntary.




About the study
Our goal is to identify effective, safe, affordable, and evidence-based medications so they can be accessed by the community
Help people with COVID-19 feel better faster, stay out of the hospital, and prevent long COVID
Open to adults aged 18–49 years with one or more chronic condition(s) or 50+ years who tested positive for COVID with symptoms starting within the last five days
Directly influence standards of care for COVID-19 infection in community settings in Canada and around the world
Supported by the Canadian Institutes of Health Research, Health Canada and the Public Health Agency of Canada
30+ partner organizations engaging diverse populations across Canada to evaluate the clinical- and cost-effectiveness of medications for COVID-19
Participate in our study
Participate in our study
The CanTreatCOVID trial relies on volunteers, often referred to as participants, to help us better understand the effectiveness of medications for mild to moderate COVID-19. The study is open to adults aged 18 – 49 years with one or more chronic condition(s) or adults aged 50+ years who tested positive for COVID with symptoms starting within the last five days.
COVID-19 Data Trackers
WHO COVID-19 Dashboard. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO. Available online: https://covid19.who.int/.