COVID-19 trial now enrolling participants
The study is open to adults aged 18–49 years with one or more chronic condition(s) or adults aged 50+ years who tested positive for COVID with symptoms starting in the last five days.
CanTreatCOVID is publicly funded research studying medications to see if they can help people with COVID-19 feel better faster, stay out of the hospital, and prevent long COVID.
About the study
While public health measures and vaccines have reduced the spread of COVID-19, most scientists predict that new variants will continue to emerge and become more community-based (endemic) versus global (pandemic). In addition, we need to study whether any acute treatment can prevent long COVID or post-COVID condition. CanTreatCOVID is an adaptive platform trial (APT) designed to identify effective and affordable medications so they can be made readily available in community settings.
CanTreatCOVID is a non-profit study supported by the Canadian Institutes of Health Research, Health Canada and the Public Health Agency of Canada.
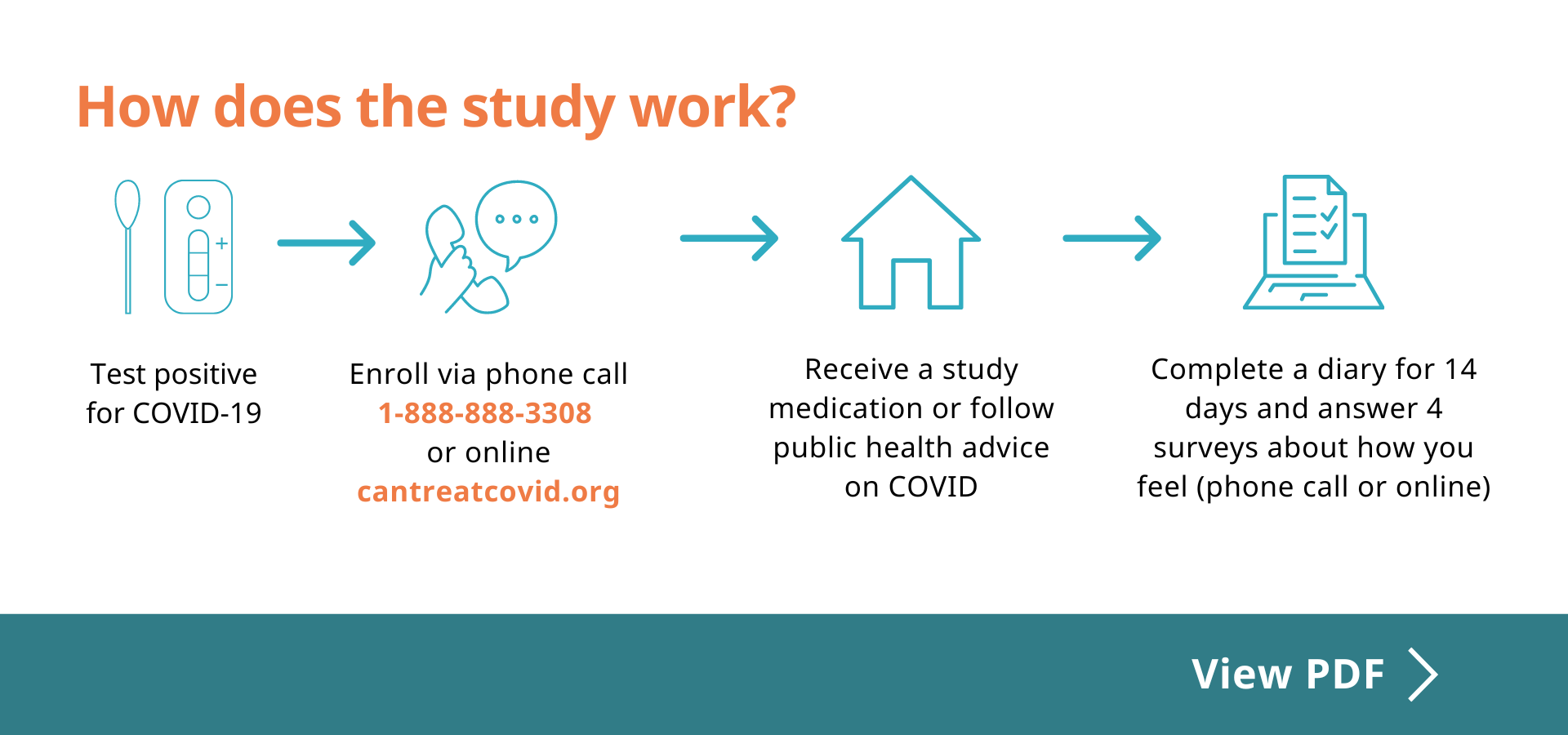
The CanTreatCOVID trial relies on volunteers, often referred to as participants, to help us better understand the effectiveness of medications for mild to moderate COVID-19.
The study is open to
- Adults aged 18-49 years with one or more chronic condition(s) listed below OR adults aged 50+ years
- Who tested positive for COVID-19 with symptoms starting within the last five days and
- Living in one of the following provinces: Ontario, Quebec, British Columbia, Alberta, Manitoba, or Newfoundland and Labrador.
Individuals currently taking Paxlovid or hospitalized for COVID-19 are not eligible to participate.
Adults aged 18-49 years who tested positive for COVID must have at least one of the following chronic conditions to participate in the study:
- Chronic respiratory disease (including COPD, cystic fibrosis and asthma requiring at least daily use of preventative and/or reliever medication)
- Chronic heart or vascular disease
- Chronic kidney disease
- Chronic liver disease
- Chronic neurological disease (including dementia, stroke, epilepsy)
- Severe and profound learning disability
- Down Syndrome
- Diabetes (Type 1 or Type 2)
- Immunosuppression: primary (e.g. inherited immune disorders resulting from genetic mutations) or secondary due to disease or treatment (e.g. sickle cell, HIV, cancer, chemotherapy)
- Solid organ, bone marrow and stem cell transplant recipients
- Morbid obesity (BMI >35)
- Severe mental illness
- Care home resident
Click here to complete the pre-screening form
We will recruit patients by public communications and through primary care clinics, out-patient clinics, and emergency departments, and by using Electronic Medical Record (EMR) data from primary care research networks in Ontario, Quebec, Alberta, British Columbia, Manitoba and Newfoundland.
COVID-19 vaccines have helped to reduce infection severity in Canada and around the world. As a result, patients with mild to moderate symptoms are now going to their primary care providers, including family doctors, walk-in clinics, and other community care settings, for access to care for COVID-19.
Although their symptoms may not be as severe, some patients can still be at risk of hospitalization, death, or developing long COVID. It is urgent that we identify effective, safe, affordable, and evidence-based medications so they can be accessed by the community, reducing the burden on hospitals and ICUs. The long-term goal of the study is to find medications that prevent long COVID, a condition that affects some people even after they recover from the virus.
Usually, clinical research trials studying medications only involve very few treatments. As researchers are continuously working on innovating existing medications and developing new ones for COVID-19, CanTreatCOVID needed to use a study design that would let us study multiple medications over time. Adaptive platform trials (APTs) allow research teams to get approval for a ‘master’ protocol, outlining in detail study procedures and methods, but then add new medications to the trial as they emerge without having to start from the beginning.
During the COVID-19 pandemic, APTs have been crucial in identifying what does and does not work to treat COVID-19 among hospitalized patients, and we would like to replicate this success at the community level.
A transparent Canadian COVID-19 Out-Patient Therapeutics Committee will identify the medications to be investigated in the CanTreatCOVID study.
We will measure the drug efficacy by comparing hospitalization and death at 28 days, as well as time to recovery and impact on long COVID. We will also assess changes in quality of life and the usage of health resources to evaluate the cost-effectiveness of each medication and/or combination of medications.
Findings will be shared through publications, media channels, newsletters, social media, and to policymakers and healthcare providers across Canada.
To ensure the findings are shared widely and effectively, the CanTreatCOVID team will also work closely with APTs in the United Kingdom and European Union.
Participants who are enrolled in the trial will be randomized to receive either a study drug or usual care. Study drugs will be those identified by the Canadian COVID-19 Out-Patient Therapeutics Committee and approved by research ethics to be evaluated in this trial. Usual care involves supportive care (i.e., rest and fluids) and symptom relief.
Usual care is included in this trial because we want to know if potential medications for COVID-19 are more effective at reducing outcomes such as hospitalizations, death, severity and duration of symptoms, and the occurrence of long COVID than resting, taking fluids, and taking something to relieve symptoms, if necessary.
The results of this study will help Canada and other countries in deciding which treatments are most effective in reducing hospitalization and death among patients with COVID-19, while also being cost-effective, adding to our current knowledge on medications and therapeutics during the COVID-19 pandemic. Our findings will directly influence standards of care for COVID-19 infection in community settings in Canada and around the world.
This study will also build experience within Canada with conducting APTs, something that is currently lacking. Beyond COVID-19, our APT infrastructure can be adapted to study therapeutics for influenza, other upper respiratory pathogens and other diseases in the future.
Frequently Asked Questions
Participants
Participants will
- Take a COVID medication approved by Health Canada or follow public health advice on COVID
- Complete an online diary from Day 1 to 14 (online or by phone call)
- Complete an online survey on Day 21, 28 and 90 and Week 36
- Receive $30 for each follow-up
There are no costs associated with participating in the study. If participants are randomized to the treatment arm, they will receive the study drug for free.
Participants will receive $30 for each follow-up (Day 21, 28, 90 and Week 36).
Referral
- Anyone can refer adults with a positive COVID test who are aged 50+ years or 18-49 years with one or more chronic condition(s).
- Participants may also contact the study team directly.
To refer participants, ask them to
- Call 1-888-888-3308 (Monday – Friday, from 8 am – 6 pm Eastern Time) or
- Email info@CanTreatCOVID.org or
- Visit https://redcap.link/CanTreatCOVID
For primary care providers:
- This will help you save time. You can refer adults who tested positive for COVID to our study, and we will screen if they are eligible to receive COVID medications, including nirmatrelvir/ritonavir (Paxlovid).
- This study is the fastest way to answer whether these medications are effective, particularly in a highly vaccinated population.
- This is by primary care providers, for primary care providers! CanTreatCOVID is helping us launch the new Canadian Primary Care Trials Network, finally creating evidence in the real world of primary care.
Trial Screening Assessment
We will conduct a risk assessment to verify that individuals meet the study eligibility criteria and are able to receive the study drug, including Paxlovid. This process will consist of three steps:
Step One: Research Assistant Screening
The research assistant will go through a series of questions with potential participants to determine if they meet the inclusion and exclusion criteria for the study. These questions include demographic information, current health status, and any previous medical conditions or treatments.
Step Two: Medication Review by Study Pharmacist
If participants are potentially eligible, they will then be connected with a study pharmacist who will review their current medication list to ensure that they are not taking any prohibited drugs. This will help to ensure that any potential interactions or contraindications with the study medication are identified before the participant is enrolled.
Step Three: Final Eligibility Review by Study Physician
As the final step, the study physician will review the participant’s medical history and medication history to confirm their eligibility. The physician will also ensure that the participant meets the inclusion and exclusion criteria and that they have provided informed consent to participate in the study.
This process helps to ensure that only eligible and suitable participants are enrolled in the study and that the results of the study are accurate and reliable. It also helps to protect the safety and well-being of the participants.
Additional Exclusion Criteria for Paxlovid
Paxlovid is contraindicated for use in individuals who:
- are pregnant or have a known or suspected pregnancy
- are breastfeeding
- have the potential to bear children and are unwilling to use effective contraception
- have a history of hypersensitivity to nirmatrelvir/ritonavir or any of its excipients
- have galactose intolerance, lactase deficiency, or glucose-galactose malabsorption
- have severe liver impairment
- have moderate or severe renal disease (defined as CKD stage 3, 4 or 5 or current acute kidney injury or most recent eGFR in the past 6 months <60 ml/min)*
- are currently taking Paxlovid
- are taking a drug that is contraindicated or not recommended for use with Paxlovid
* Please note that individuals with a GFR of 30-60 ml/min must take a reduced dose of Paxlovid and will not be eligible to participate in the CanTreatCOVID study as all participants must receive the same dose.
After Enrollment of Participants
- If participants are randomized to the treatment arm, we will ship the study drug to the participants for free.
- Shipment of the study drug takes approximately 24 hours
- Participants will be closely monitored by a healthcare team, which helps to ensure that any side effects or complications are identified and addressed quickly.
- Participation in this study provides patients with personalized care and attention, as well as access to specialized resources and support.
Participants may decide to complete the study treatment course.
Depending on the specific circumstances, based on recommendations from the study pharmacist, the study physician may decide to end treatment for certain participants, but participants may still be asked to continue completing daily diaries and participating in follow-up phone calls to provide ongoing data for the study.
Yes, CanTreatCOVID is an open-label study and participants will know if they are randomized to receive the study drug or follow usual care recommendations from public health.
- Yes, the study forms and questionnaires are available in French and English and if needed, additional languages will be added to accommodate study participants.
- If potential participants are unable to communicate in English or French, the study staff may refer the consenting process to someone within the study team that speaks the required language or ask to speak with an alternate contact who is able to communicate in English or French.
On December 1, 2022, Health Canada authorized an extension to the shelf-life of Paxlovid. Depending on the lot number, the expiry date has been extended by 6 months to 24 months. If you are a CanTreatCOVID study participant and have been allocated to one of the active treatment groups, call our toll-free number (1-888-888-3308) to consult with a pharmacist if you have any questions regarding your medication. Refer to this fact sheet for more information.
Significance of the Study
- Existing studies have been in unvaccinated patients. It is unclear whether and to what extent existing treatments (including Paxlovid) are effective in partially or fully vaccinated patients.
- Existing knowledge about COVID treatments (including Paxlovid) is mostly based on observational studies. We need high-quality evidence to further understand the effectiveness of COVID medications.
- Currently, no treatment has been evaluated specifically for its potential to prevent long-term symptoms of COVID (long COVID).
Patients may choose to participate in a study for several reasons:
- Close monitoring: In this study, patients will be closely monitored by a healthcare team, which helps to ensure that any side effects or complications are identified and addressed quickly.
- Personalized care: Participation in this study provides patients with personalized care and attention, as well as access to specialized resources and support.
- No cost: We cover the cost of treatment and related expenses, so patients do not have to pay for their care.
- Contribution to medical research: By participating in this study, patients are contributing to the identification of the most effective therapeutics for non-hospitalized COVID-19 patients, advancing our understanding of the disease and improving treatment options for future patients.
It’s important to consider that participating in this study is voluntary and that patients can always decide not to participate or to withdraw from the study at any time.
CanTreatCOVID will go beyond the administration of nirmatrelvir/ritonavir (Paxlovid) for 5 days, and additional therapeutics will be added as determined by the Trial Scientific Committee.
Our partners
Together we can treat COVID.
Together we can treat COVID.
The CanTreatCOVID study is based at MAP Centre for Urban Health Solutions, Unity Health Toronto, and supported by the Canadian Institutes of Health Research (CIHR), Health Canada, Public Health Agency of Canada, and our educational, clinical and research partners from across Canada.